Driving and Pioneering Clinical Research
Academic Institutions
Academic institutions promote medical research and play a crucial role in educating future healthcare providers and developing innovative treatment methods. It is essential that these institutions have advanced tools to conduct clinical research efficiently while complying with strict regulations. ResearchManager offers academic institutions a comprehensive range of tools and setups to support them in enhancing their research and promoting collaboration both within and outside the institution. Explore the possibilities here!

Optimizing Research Processes with CTMS/eTMF
In academic institutions, conducting clinical research is often an integral part of the overall academic process. ResearchManager CTMS and eTMF provide integrated tools that empower academic institutions to streamline clinical research processes and organize documentation. Whether it’s setting up studies, managing standards and requirements, selecting and managing research sites, or monitoring finances, these tools enable academic researchers and administrators to collaborate efficiently and effectively.
Accurate Data Collection with EDC
ResearchManager’s Electronic Data Capture (EDC) system annually supports numerous academic researchers and students in efficiently capturing and managing accurate and reliable clinical data. Crucial for research across various academic disciplines.

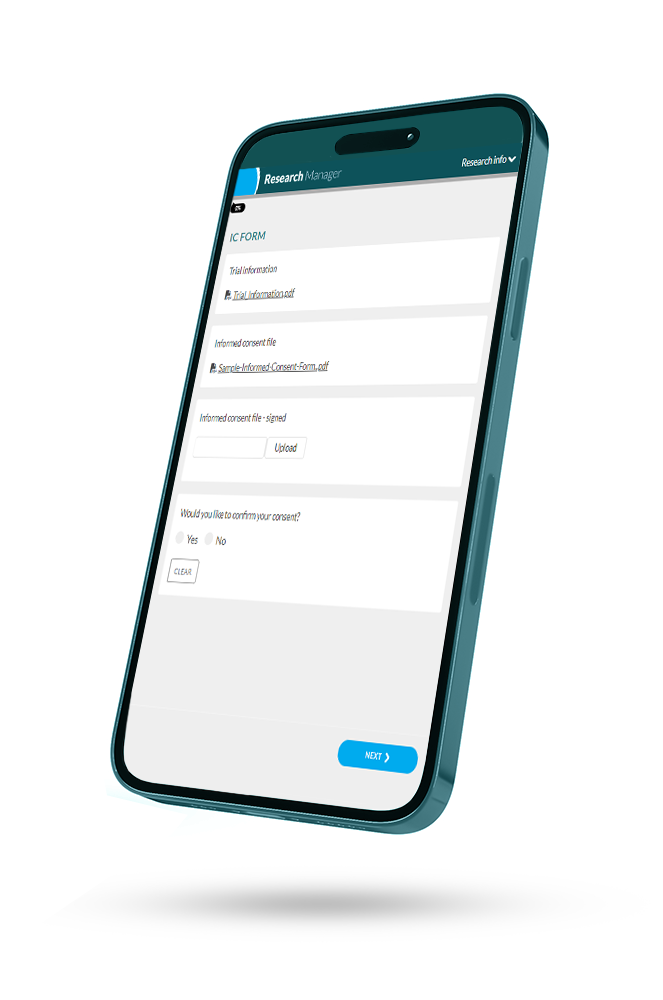
Improved Patient Engagement with ePRO and eConsent
Academic institutions are involved in research related to the health and well-being of patients. Electronic Patient-Reported Outcomes (ePRO) and Electronic Informed Consent (eConsent) are tools designed to enhance participant engagement in clinical research. ePRO allows students and researchers to capture data on patients’ health and well-being in a user-friendly manner. eConsent simplifies the consent process, leading to smoother participant recruitment and better adherence to ethical standards. These tools strengthen the relationship between academic researchers and participants, ultimately enhancing the quality of research and education within academic institutions.
Users and Partners of ResearchManager

Want to Learn More About Pricing?
Reviews

Thierry Wetting
International Sales Manager