Streamlining Consent Process Digitally
electronic Consent
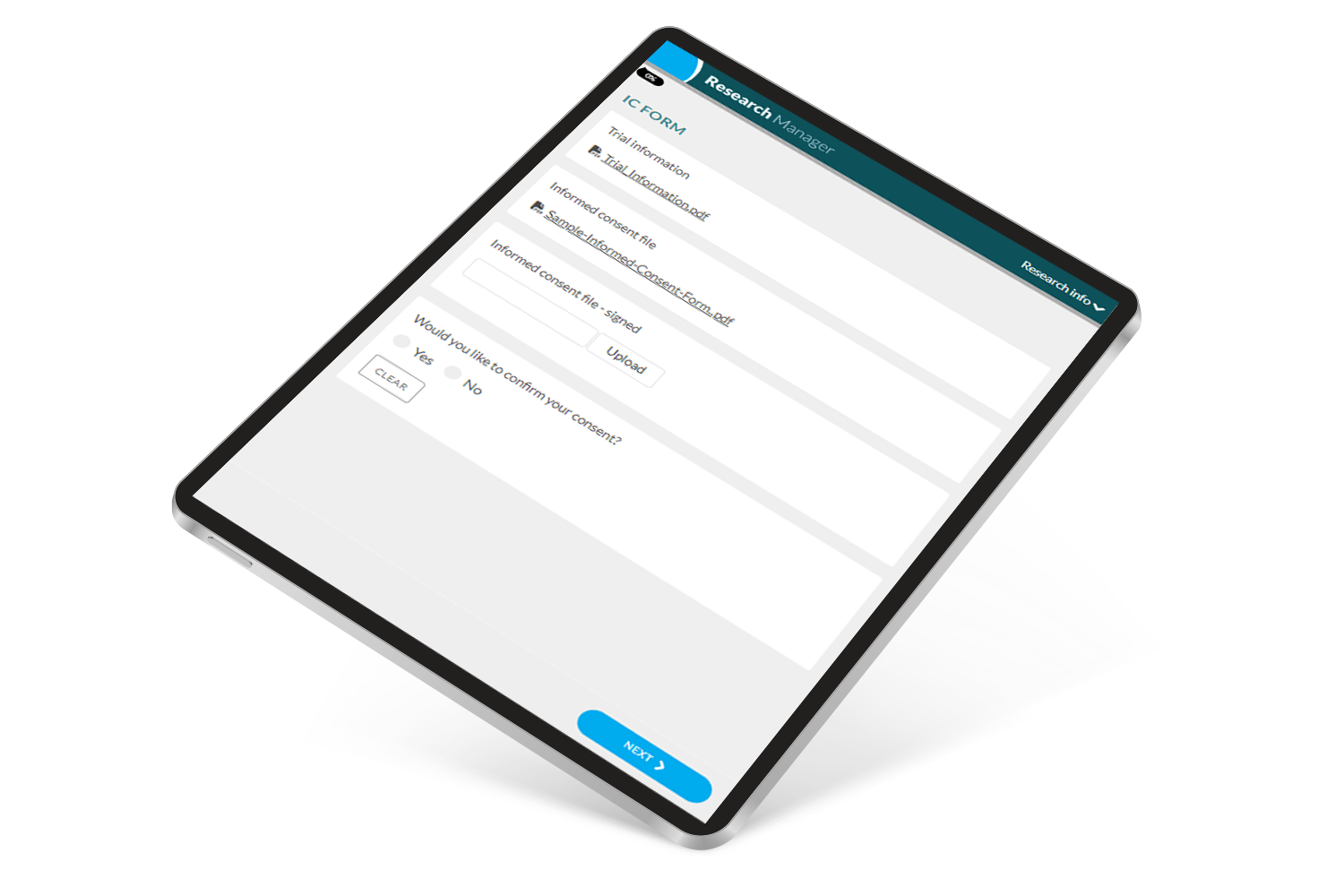
Participant engagement is crucial for the success of a clinical trial. ResearchManager eConsent fosters participant engagement in a positive manner while maintaining the integrity of the research. Instead of scattered paperwork like consent forms, eConsent provides an organized and accessible solution.

Information Flow and Participant Consent
ResearchManager eConsent provides participants with the ability to digitally access essential research information before consenting to participate. This not only promotes transparency but also ensures a better understanding of the research, realistic expectations, and an improved participant experience. As a result, research teams can manage the consent process efficiently and ethically.
eConsent Increases Engagement
Information is key, especially in clinical research. Active engagement is crucial. With multimedia tools such as explanatory videos, participants can easily access essential information about their research and involvement. This enhances satisfaction and results in better and more comprehensive responses.

eConsent Meets All Legal Requirements
Working with participants entails additional requirements from the law for patient safety and secure data management. eConsent is secure and complies with regulatory standards and legislation, such as GCP. Additionally, eConsent provides documentation of the consent process, including digital signatures, audit trails, and secure data storage. This ensures that the research meets submission and inspection requirements, and enables monitoring. Therefore, eConsent safeguards the integrity of the research.
Users and Partners of ResearchManager

Want to Learn More About Pricing?
Compliance and Technical Information
ResearchManager is compliant
- Offices in the Netherlands (HQ) and in the USA.
- ISO 27001 certified
- NEN 7510 certified
- ISO 14155 compliant
- 21 CFR PART 11 compliant
- GCP compliant
- GDPR compliant
- HIPAA compliant
- Secure connection with Comodo’s EV SSL certificate
Information about our Data Centers
- TIER 3+ data centers in the EU
- SOC 2 security standards
- ISO 9001 certified
- ISO 14155 certified
- ISO 27001 certified
- NEN 7510 certified
- Dedicated backup server, AES 256 encrypted

Thierry Wetting
International Sales Manager
Ready to see more?
Book a free demonstration with one of our specialists using the button below. We'll guide you through and demonstrate how our solutions can support you.